REMSleep Outlines Q1 2026 Commercial Strategy: Expanded FDA Application, B2B Launch Prep, and Institutional Market Entry
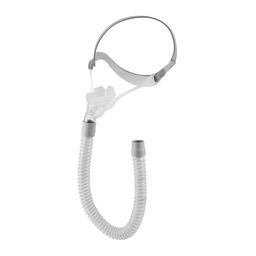
The revolutionary REMsleep DeltaWave mask.
Company Transitions from Infrastructure Buildout to Active Market Execution as Expanded 510(k) Review Enters Final Stages
REMSleep Holdings, Inc. (OTCQB:RMSL)
BLACKSHEARS, GA, UNITED STATES, January 6, 2026 /EINPresswire.com/ -- REMSleep Holdings, Inc. (OTCQB: RMSL), developer of the FDA-cleared DeltaWave™ CPAP mask system, today provided shareholders with a strategic outlook for Q1 2026 as the company transitions from foundational infrastructure work to full commercial execution.
After spending 2025 building operational systems, manufacturing capabilities, and distribution networks, REMSleep enters the new year with what CEO Thomas Wood described as "all the pieces finally in place to run this thing properly."
The past two weeks of updates detailed a 20-person nationwide sales force, enterprise inventory management systems, replacement parts supply chains, and early market validation. Now the company is outlining how those assets were deployed in the first quarter.
**Expanded 510(k) Application:**
In early December, REMSleep submitted a supplemental 510(k) application to the FDA, expanding DeltaWave's indicated use beyond traditional CPAP therapy. The application seeks clearance for use with BiPAP and other positive pressure ventilation devices, as well as approval for institutional single-patient use in hospital settings.
The original 510(k) clearance was narrowly focused on home-use CPAP therapy, leaving COPD patients on non-invasive ventilation and hospital respiratory therapy applications off the table.
"Whoever handled the original 510(k) filing was overly conservative with the language," said Jeff Marshall, REMSleep's operations manager. "They kept saying 'CPAP, CPAP, CPAP,' which is fine, but DeltaWave's core design features—especially CO2 rebreathing reduction—are arguably more valuable for patients on BiPAP or ventilation support."
The supplemental filing leverages the existing 510(k) clearance and is being handled by a regulatory consulting firm that works with major manufacturers like Philips and ResMed. Based on FDA timelines for this type of application, REMSleep expects a response by early to mid-January.
If approved, the expanded clearance opens institutional sales channels with higher price points than home care markets. It also positions DeltaWave for clinical settings where buying decisions are made by pulmonologists and respiratory therapists.
**Three-Pronged Sales Approach:**
REMSleep's go-to-market strategy for Q1 focuses on three distinct channels, each with different risk profiles and sales cycles:
1. **DME/HME "Rescue Mask" Positioning:** The company is targeting the 20-30% of new CPAP patients who fail their initial mask interface and require a second option. Rather than asking DME providers to overhaul their primary formularies, sales reps are positioning DeltaWave as the backup option when standard masks don't work. Early tests have shown receptivity from providers.
2. **Internet Provider Partnerships:** Two online CPAP retailers are already on their second and third orders after initial trials. These providers operate outside traditional DME reimbursement constraints and can move faster on product adoption. REMSleep is expanding discussions with additional internet-based distributors.
3. **Institutional Sales (pending expanded 510(k)):** If the supplemental FDA application is approved, REMSleep will activate outreach to hospital respiratory departments, long-term care facilities, and rehabilitation centers. This channel requires longer sales cycles but offers bulk purchasing potential and reduces dependence on insurance reimbursement complexity.
**Sustainable Volume Targets:**
Rather than projecting explosive growth, REMSleep is focused on reaching what Marshall called a "float threshold"—monthly unit volumes that generate sufficient cash flow to support operations while broader marketing initiatives and partnerships develop.
The company is targeting 1,500-1,600 masks per month as the initial sustainable benchmark. At that volume, REMSleep can maintain operations and continue funding product development without constant capital raises.
"We're not trying to be a unicorn by March," Wood said. "We're trying to prove this business can operate, generate cash, and scale methodically. If we get to 1,500 units a month by end of Q1, that changes the entire conversation about what comes next."
**What 2025 Taught Management:**
Wood acknowledged that REMSleep's 2025 communications with shareholders were inconsistent. The October press release suggested the company was further along than it was; subsequent months were silent as management worked through operational challenges.
"I learned that being quiet while you fix problems just creates more problems," Wood said. "The last several weeks of weekly updates have generated more positive feedback than anything we did in the prior six months combined."
The company committed to maintaining regular operational updates throughout Q1 with specific progress metrics rather than generic forward-looking statements.
**Capital Strategy:**
REMSleep is exploring multiple paths to secure growth capital in Q1, including traditional private placements and strategic partnerships.
"Nobody wants to fund a concept anymore," Wood said. "They want to fund traction. Our job in Q1 is to create enough traction that the capital conversation shifts from 'why should we take this risk' to 'how do we get a piece of this.'"
**Looking Ahead:**
REMSleep enters 2026 with infrastructure complete, initial market validation in hand, and a clear execution roadmap. The company expects Q1 to be a defining period.
"We've spent four years and a lot of money getting here," Wood said. "The product works. The infrastructure works. Now it's just execution. Q1 2026 is when we find out if all this work was worth it."
**About REMSleep Holdings, Inc.**
REMSleep Holdings, Inc. (OTCQB: RMSL) is a medical device company focused on improving outcomes for patients requiring positive airway pressure therapy. The company's DeltaWave™ system is FDA-cleared and designed to address common compliance challenges in CPAP therapy through its patented interface technology.
**Forward-Looking Statements**
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements involve risks and uncertainties that could cause actual results to differ materially from those expressed or implied, including but not limited to: FDA regulatory review timelines, market acceptance of the DeltaWave system across multiple channels, the company's ability to achieve targeted sales volumes, the development of manufacturing and distribution partnerships, capital raising activities, and general market conditions. REMSleep undertakes no obligation to update these forward-looking statements.
Investor Relations
REM Sleep Holdings Inc
+1 912-590-2001
email us here
Visit us on social media:
LinkedIn
Instagram
Facebook
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.