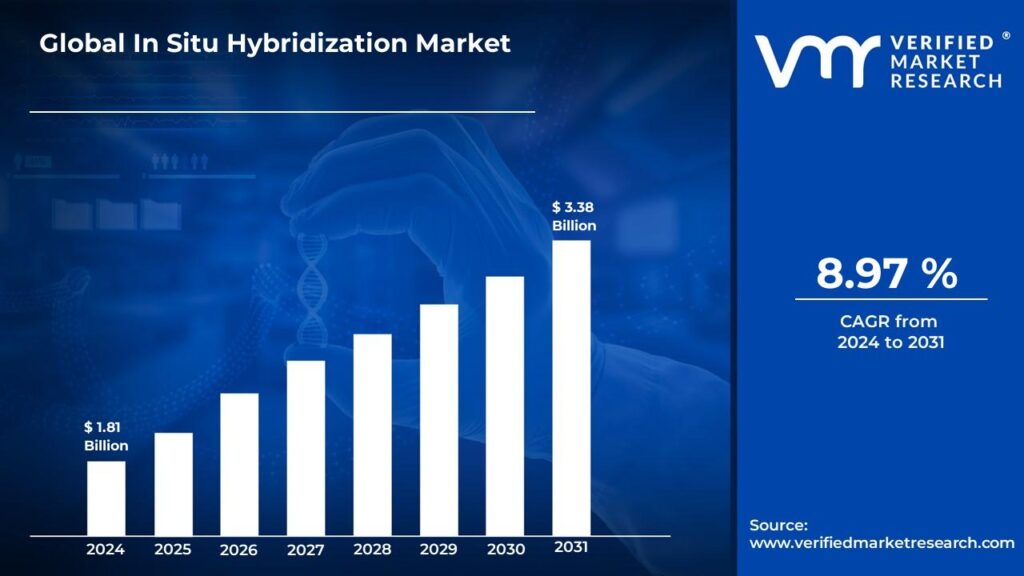
In Situ Hybridization Market is expected to generate a revenue of USD 3.38 Billion by 2031, Globally, at 8.97% CAGR: Verified Market Research®
The In Situ Hybridization market presents robust growth opportunities driven by rising cancer prevalence, demand for personalized medicine, and technological advancements. However, high operational costs, procedural complexity, and skill shortages pose entry barriers. North America’s dominance underscores the value of targeting developed markets with advanced infrastructure and regulatory clarity. For new entrants and existing players, focusing on automation-driven ISH platforms, training services, and strategic partnerships in high-growth regions like Asia-Pacific can unlock scalability. A value-driven, innovation-led approach tailored to diagnostic labs and research institutions will be key to capturing long-term market share.
Lewes, Delaware, July 14, 2025 (GLOBE NEWSWIRE) -- The Global In Situ Hybridization Market Size is projected to grow at a CAGR of 8.97% from 2024 to 2031, according to a new report published by Verified Market Research®. The report reveals that the market was valued at USD 1.81 Billion in 2024 and is expected to reach USD 3.38 Billion by the end of the forecast period.
The In Situ Hybridization Market is experiencing rapid growth as it plays a crucial role in detecting genetic abnormalities and disease markers at the cellular level. Rising investments in biotech R&D and demand for accurate tissue-based diagnostics are fueling its global adoption.
Key Highlights of the Report:
- Market Size & Forecast: In-depth analysis of current value and future projections
- Segment Analysis: Detailed study Technique, Application, and End User.
- Regional Insights: Comprehensive coverage of North America, Europe, Asia-Pacific, and more
- Competitive Landscape: Profiles of top players and their strategic initiatives
- Rising Demand in Oncology Research: Surge in cancer cases is driving adoption of ISH for biomarker analysis.
- Growth in Personalized Medicine: Precision diagnostics are boosting ISH use in targeted therapies.
- Technological Advancements: Automation and digital pathology are enhancing ISH efficiency.
- High Procedural Costs: Expensive reagents and instruments limit access in cost-sensitive regions.
-
Challenges and Risk Assessment: Evaluates ethical debates, off-target effects, and regulatory complexities.
Why This Report Matters:
This report delivers in-depth insights into current and future trends, enabling stakeholders to make informed investment, R&D, and market entry decisions. It offers a complete view of the competitive landscape, technology trends, regulatory frameworks, and growth opportunities across regions.
Who You Should Read This Report:
- Biotechnology & Pharma Companies exploring diagnostic tool expansion
- Investors & Venture Capitalists identifying emerging molecular diagnostics markets
- Healthcare Institutions & Labs evaluating new pathology techniques
- Academic & Research Institutes engaged in molecular and cancer research
-
Market Research Professionals seeking credible and detailed market intelligence
 For more information or to purchase the report, please contact us at: https://www.verifiedmarketresearch.com/download-sample/?rid=24153
For more information or to purchase the report, please contact us at: https://www.verifiedmarketresearch.com/download-sample/?rid=24153
Browse in-depth TOC on “Global In Situ Hybridization Market Size”
202 - Pages
126 – Tables
37 – Figures
Report Scope
| REPORT ATTRIBUTES | DETAILS |
| Study Period | 2021-2031 |
| Growth Rate | CAGR of ~8.97% from 2024 to 2031 |
| Base Year for Valuation | 2024 |
| Historical Period | 2021-2023 |
| Quantitative Units | Value in USD Billion |
| Forecast Period | 2024-2031 |
| Report Coverage | Historical and Forecast Revenue Forecast, Historical and Forecast Volume, Growth Factors, Trends, Competitive Landscape, Key Players, Segmentation Analysis |
| Segments Covered |
|
| Regions Covered |
|
| Key Players | Thermo Fisher Scientific, Inc., Abbott, PerkinElmer, Inc., BioView, Agilent Technologies, Inc., Merck KGaA, Bio-Rad Laboratories, Inc. |
| Customization | Report customization along with purchase available upon request |
Global In Situ Hybridization Market Overview
Market Driver
Rising Prevalence of Cancer and Genetic Disorders: The global surge in chronic diseases—particularly cancer, genetic syndromes, and rare hereditary disorders—is one of the most significant drivers for the In Situ Hybridization market. ISH plays a crucial role in identifying chromosomal abnormalities, gene amplifications, deletions, and viral infections at the cellular level. It enables the visualization of molecular signals within tissue architecture, offering accurate localization of nucleic acid sequences. With cancer becoming a leading cause of death globally, healthcare providers are prioritizing early detection and precise molecular diagnostics. Governments and private healthcare institutions are investing heavily in molecular pathology labs, further expanding ISH adoption in oncology diagnostics and translational research. As the focus shifts toward biomarker-based treatments and companion diagnostics, ISH is increasingly recognized as an indispensable tool in modern diagnostics.
Expanding Applications in Personalized and Precision Medicine: Personalized medicine continues to revolutionize clinical practices across the globe. With the ability to tailor treatment protocols based on the unique genetic makeup of patients, the demand for techniques that can provide molecular insights—like ISH—is rising sharply. ISH helps determine the spatial distribution and expression level of specific RNA or DNA sequences in tissues, which is crucial in diseases such as breast cancer (HER2 testing), lung cancer (ALK/ROS1 rearrangements), and neurological disorders. Pharmaceutical companies are using ISH in preclinical and clinical studies to stratify patient populations and identify responders to targeted therapies. The increasing clinical adoption of ISH in stratifying patients for immunotherapies, gene therapies, and targeted drugs has positioned it as a vital component in personalized healthcare delivery.
Technological Advancements and Workflow Automation: Recent advancements in ISH technologies—including chromogenic in situ hybridization (CISH), fluorescence in situ hybridization (FISH), and RNAscope—are transforming the operational capabilities of pathology laboratories. Modern ISH platforms are equipped with automation, multiplexing capabilities, and high-resolution digital imaging, enabling greater accuracy, reproducibility, and throughput. Automation minimizes human intervention, reducing variability and speeding up the diagnostic process, which is particularly valuable in high-volume clinical laboratories and research facilities. Moreover, integration with digital pathology and AI-powered image analysis tools is enabling pathologists to extract richer datasets from ISH images, improving diagnostic accuracy and reducing turnaround times. These enhancements are encouraging adoption across pharmaceutical R&D, academic institutes, and central labs, driving sustained market expansion.
 To Purchase a Comprehensive Report Analysis: https://www.verifiedmarketresearch.com/select-licence/?rid=24153
To Purchase a Comprehensive Report Analysis: https://www.verifiedmarketresearch.com/select-licence/?rid=24153
Market Restraint
High Cost of Equipment, Probes, and Reagents: One of the most pressing challenges in the ISH market is the substantial capital investment required for setting up and maintaining the technology. ISH assays involve expensive fluorescent or chromogenic probes, high-end microscopes, hybridization chambers, and digital image analysis software—all of which entail significant upfront and ongoing costs. In addition to hardware and consumables, recurring operational costs related to skilled labor, calibration, and reagent replenishment increase the total cost of ownership. This becomes particularly challenging for diagnostic labs and research centers in cost-sensitive or emerging economies. While developed countries can afford to integrate ISH into routine pathology, financial constraints in public healthcare systems in developing regions remain a major roadblock to widespread adoption.
Shortage of Skilled Pathologists and Molecular Technicians: The technical complexity of ISH assays demands a workforce trained in both histopathology and molecular biology. Operators must be proficient in slide preparation, probe hybridization, signal detection, and image interpretation. However, there exists a significant global shortage of skilled professionals capable of executing ISH protocols with consistency. This skill gap often leads to variability in results, higher error rates, and underutilization of the technology. Emerging economies, in particular, suffer from a lack of training infrastructure, limiting the scalability of ISH-based services. Even in developed regions, the steep learning curve and constant evolution of techniques like multiplexed ISH present a challenge for keeping personnel updated.
Procedural Complexity and Lack of Standardization: In Situ Hybridization procedures are highly sensitive to variations in tissue preparation, probe concentration, hybridization temperature, and signal amplification. Small deviations can result in false positives or negatives, which are critical issues in clinical diagnostics. Additionally, lack of universally accepted protocols across different laboratories leads to inconsistencies in outcomes. Regulatory agencies and accreditation bodies are pushing for standardization, but disparities remain in interpretation criteria, signal quantification, and reporting guidelines. These challenges make it difficult for laboratories to ensure reproducible, high-quality results at scale. The lack of harmonized quality control measures across the industry continues to impede trust and broader adoption, especially in regulatory-sensitive applications such as companion diagnostics.
Geographical Dominance: North America holds the dominant share in the In Situ Hybridization market, driven by advanced healthcare infrastructure, high R&D spending, and widespread adoption of molecular diagnostics. The presence of leading biotech firms, academic research institutions, and clinical laboratories enhances the region's innovation pipeline. Favorable reimbursement policies and a growing focus on personalized medicine further support market expansion, making North America a lucrative hub for ISH-based diagnostics and research.
Key Players
The “Global In Situ Hybridization Market” study report will provide a valuable insight with an emphasis on the global market. The major players in the market are Thermo Fisher Scientific, Inc., Abbott, PerkinElmer, Inc., BioView, Agilent Technologies, Inc., Merck KGaA, Bio-Rad Laboratories, Inc.
In Situ Hybridization Market Segment Analysis
Based on the research, Verified Market Research has segmented the global market into Technique, Application, End-User, and Geography.
-
In Situ Hybridization Market, by Technique:
- Fluorescent In Situ Hybridization (FISH)
- Chromogenic In Situ Hybridization (CISH)
- Radioactive In Situ Hybridization (RISH)
-
In Situ Hybridization Market, by Application:
- Cancer Diagnosis and Research
- Neuroscience
- Infectious Disease Diagnosis
- Prenatal Testing
- Drug Discovery and Development
-
In Situ Hybridization Market, by End-User:
- Hospitals and Diagnostic Laboratories
- Biopharmaceutical Companies
- Academic and Research Institutes
-
In Situ Hybridization Market, by Geography
-
North America
- U.S
- Canada
- Mexico
-
Europe
- Germany
- France
- U.K
- Rest of Europe
-
Asia Pacific
- China
- Japan
- India
- Rest of Asia Pacific
-
ROW
- Middle East & Africa
- Latin America
-
North America
Browse Related Reports:
Global Esophageal Cancer Molecular Diagnosis Market Size By Technology (Polymerase Chain Reaction (PCR), Next-Generation Sequencing (NGS), Fluorescence In-Situ Hybridization (FISH), Immunohistochemistry (IHC), Microarray), By Application (Early Cancer Screening, Therapeutic Monitoring, Prognostic Testing, Recurrence Prediction), By End-User (Hospitals and Clinics, Diagnostic Laboratories, Academic and Research Institutes), By Geography, And Forecast
Global Tissue Microarray Market Size By Procedure (Immunohistochemistry (IHC), Fluorescence In Situ Hybridization (FISH)), By Technology (Polymerase Chain Reaction (PCR), Amplifies DNA for Detection, Next-Generation Sequencing (NGS)), By End-User (Pharmaceutical and Biotechnological Companies, Research Organizations), By Geography, And Forecast
Global Tissue Diagnostics Market Size By Disease (Prostate Cancer, Gastric Cancer), By Technology (Immunohistochemistry (IHC), In Situ Hybridization (ISH)), By End-User (Hospitals, Research Laboratories), By Geography, And Forecast
Global Litter Treatment Market Size By Functionality (Ammonia Control, Pathogen Load Reduction), By End-User (Poultry Farms, Livestock Farms), By Product Type (Chemical Treatments, Biological Treatments), By Application Method (In-Situ Treatment, Pre-Treatment), By Geography, And Forecast
7 Leading Cancer Therapeutic Companies batting against deadly disease
Visualize In Situ Hybridization Market using Verified Market Intelligence -:
Verified Market Intelligence is our BI Enabled Platform for narrative storytelling in this market. VMI offers in-depth forecasted trends and accurate Insights on over 20,000+ emerging & niche markets, helping you make critical revenue-impacting decisions for a brilliant future.
VMI provides a holistic overview and global competitive landscape with respect to Region, Country, Segment, and Key players of your market. Present your Market Report & findings with an inbuilt presentation feature saving over 70% of your time and resources for Investor, Sales & Marketing, R&D, and Product Development pitches. VMI enables data delivery In Excel and Interactive PDF formats with over 15+ Key Market Indicators for your market.
About Us
Verified Market Research® stands at the forefront as a global leader in Research and Consulting, offering unparalleled analytical research solutions that empower organizations with the insights needed for critical business decisions. Celebrating 10+ years of service, VMR has been instrumental in providing founders and companies with precise, up-to-date research data.
With a team of 500+ Analysts and subject matter experts, VMR leverages internationally recognized research methodologies for data collection and analyses, covering over 15,000 high impact and niche markets. This robust team ensures data integrity and offers insights that are both informative and actionable, tailored to the strategic needs of businesses across various industries.
VMR's domain expertise is recognized across 14 key industries, including Semiconductor & Electronics, Healthcare & Pharmaceuticals, Energy, Technology, Automobiles, Defense, Mining, Manufacturing, Retail, and Agriculture & Food. In-depth market analysis cover over 52 countries, with advanced data collection methods and sophisticated research techniques being utilized. This approach allows for actionable insights to be furnished by seasoned analysts, equipping clients with the essential knowledge necessary for critical revenue decisions across these varied and vital industries.
Verified Market Research® is also a member of ESOMAR, an organization renowned for setting the benchmark in ethical and professional standards in market research. This affiliation highlights VMR's dedication to conducting research with integrity and reliability, ensuring that the insights offered are not only valuable but also ethically sourced and respected worldwide.
Follow Us On: LinkedIn | Twitter | Threads | Instagram | Facebook

Mr. Edwyne Fernandes Verified Market Research® US: +1 (650)-781-4080 US Toll Free: +1 (800)-782-1768 Email: sales@verifiedmarketresearch.com Web: https://www.verifiedmarketresearch.com/ SOURCE – Verified Market Research®
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
